gammaCore™ nVNS was granted Breakthrough Device Designation for PTSD by the US Food and Drug Administration in January 2022.
Ongoing research continues to show promise for nVNS as a safe and effective option for the treatment of PTSD-related symptoms. Click the button below to be redirected to the Vagus Nerve Society website, where you can download the Clinical Practice Guidelines directly.
Background and Scope of the Problem
Understanding PTSD
- PTSD is a debilitating disorder triggered by experiencing or witnessing a traumatic event
- Characterized by intrusive thoughts, avoidance, emotional blunting, negative cognition, sleep disturbances, and hyperarousal
- In one study, 59% of patients with PTSD had severely impaired quality of life, with broad effects seen across many quality-of-life domains, including mood, social and family relationships, leisure activities, overall sense of well-being, and overall life satisfaction1
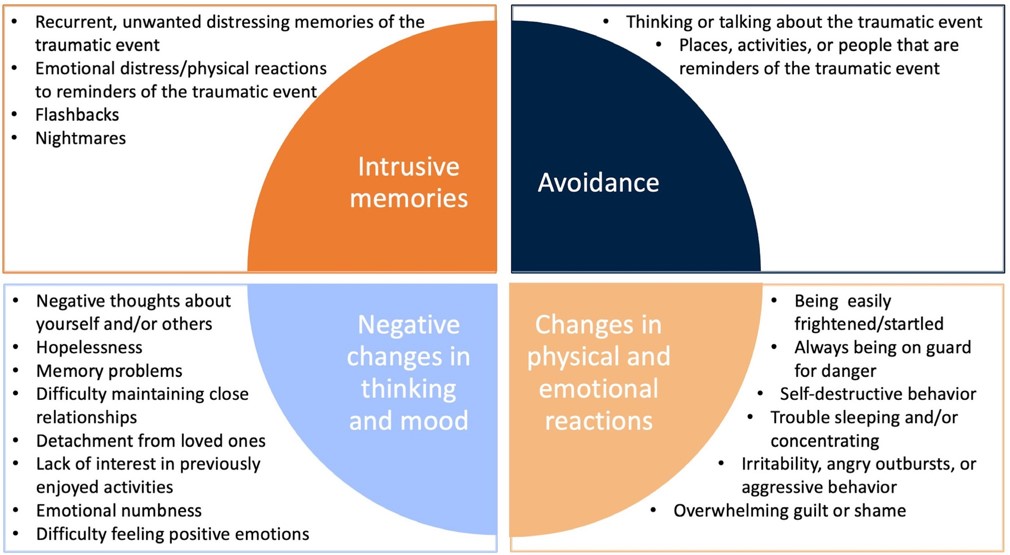
- Symptoms are grouped into 4 types:
- Intrusive memories
- Avoidance
- Negative changes in thinking and mood
- Changes in physical and emotional reactions
PTSD Symptom Categories2
Background
Different Types of Trauma That May Contribute to PTSD3
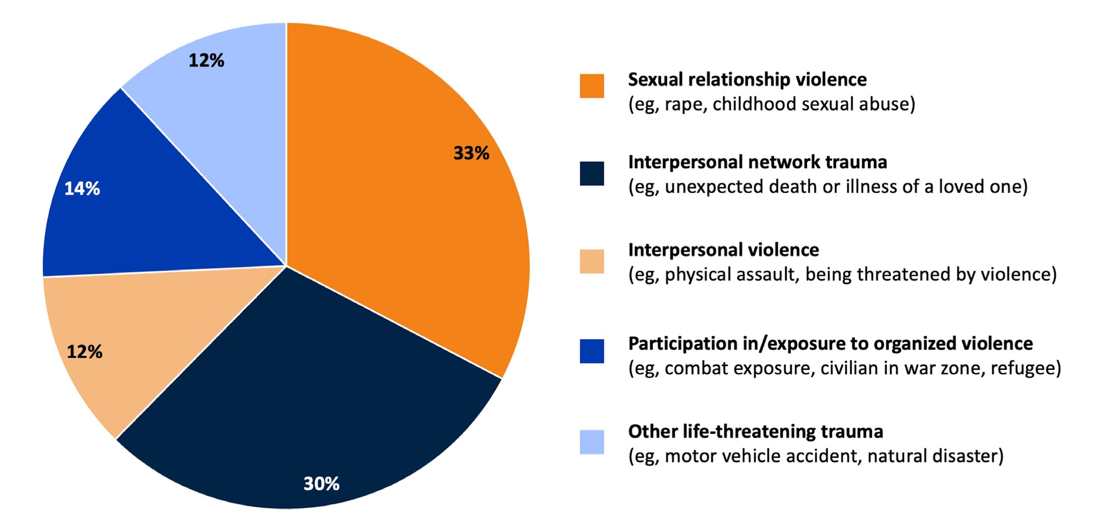
Percentages sum to >100 owing to rounding.
Scope of the Problem
- PTSD is frequently comorbid with several other conditions, including3-5
- Neurological disorders (e.g., migraine, traumatic brain injury [TBI])
- Psychiatric disorders (e.g., depression, anxiety, substance abuse)
- Cardiovascular disease (e.g., myocardial infarction, stroke, arrhythmia)
- Autoimmune and endocrine diseases (e.g., Addison disease, Guillain-Barré syndrome, immunoglobulin A nephropathy)
- Gastrointestinal disorders (e.g., irritable bowel syndrome)
Epidemiology
- PTSD has a worldwide point prevalence of 26.5%6
- Approximately 15 million adults in the US will have PTSD during a given year7
- PTSD is common among Veterans
- In a given year, an estimated 11% to 20% of Veterans of Operation Iraqi Freedom, Operation Enduring Freedom, or Operation Desert Storm have PTSD8
- Approximately 30% of Vietnam War Veterans are estimated to have had PTSD during their lifetime8
- History of a military-related mild TBI has been found to increase the odds of a PTSD diagnosis by 300%9
Neurobiology of PTSD
PTSD is a complex condition that is caused by the dysregulation of and interplay among several mechanisms of action.
Neurobiology of PTSD10
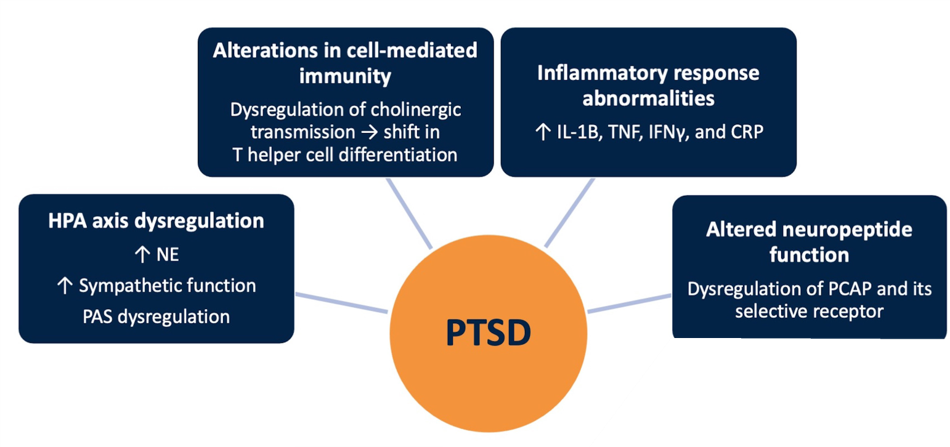
Abbreviations: CRP, C-reactive protein; HPA, hypothalamic-pituitary-adrenal; IFN, interferon; IL, interleukin; NE, norepinephrine; PAS, peripheral autonomic nervous system; PCAP, pituitary adenylate cyclase-activating polypeptide; PTSD, posttraumatic stress disorder; TNF, tumor necrosis factor.
Vagus Nerve and nVNS
Overview
- The vagus nerve integrates many functions of the central nervous system and peripheral organs
- Vagal afferents relay information from the periphery to the brain
- Vagal efferents modulate parasympathetic nervous system function in the periphery
- Human and animal research has identified several mechanistic effects of Vagus Nerve Stimulation (VNS) that are considered relevant to stress-related disorders, including PTSD
- Behavioral research in animal models that used conditioned fear stimuli indicates that VNS mitigates fear and freezing responses and can reduce anxiety24-26
- Non-invasive vagus nerve stimulation (nVNS) represents the most complete and practical application of VNS, allowing for broader clinical investigation and use than other forms of VNS
nVNS Effects Relevant to Stress-Related Disorders10-23
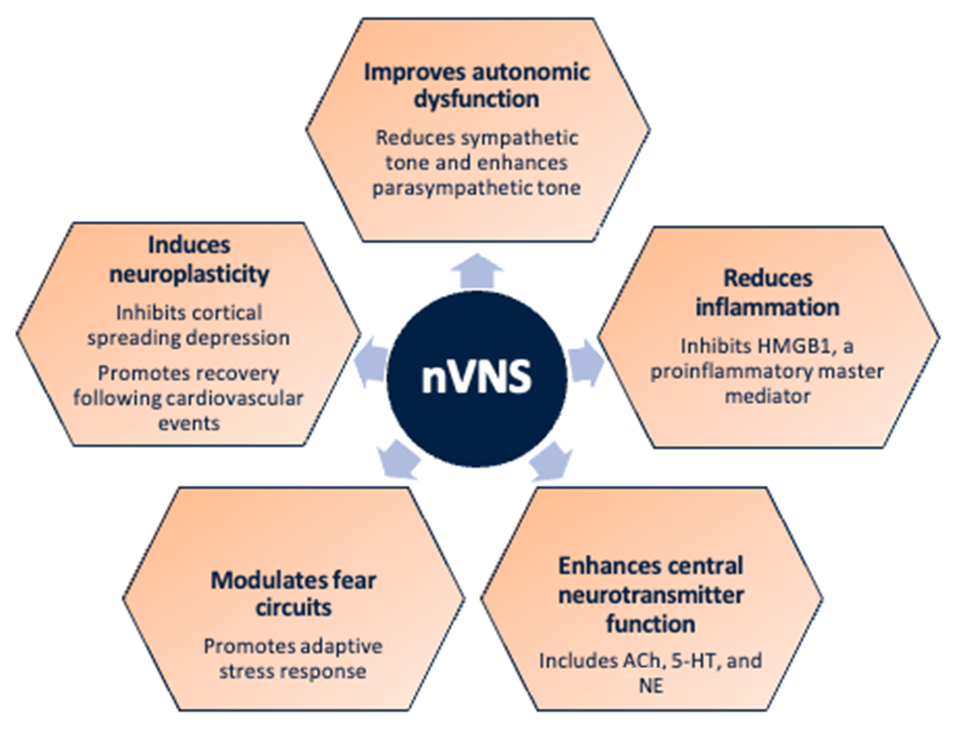
Abbreviations: 5-HT, serotonin; ACh, acetylcholine; HMGB1, high-mobility group box 1 protein; NE, norepinephrine; nVNS, non-invasive vagus nerve stimulation.
Preclinical Evidence
nVNS for prevention/treatment of comorbid TBI and PTSD27-28
- Mice exposed to blast forces modeled on those encountered in combat demonstrated increases in stress-related maladaptive behaviors, which were ameliorated when nVNS was delivered 1 hour after blast exposure
- These findings suggest a potential therapeutic role for nVNS after blast-induced mild TBI
Representative Traces of Mouse Locomotion (Purple Lines) During the Novel Object Exploration Test (A), Percentage of Time Spent With a Novel Object (B), and Latency to Interact With a Novel Object (C) 30 Days Postblast
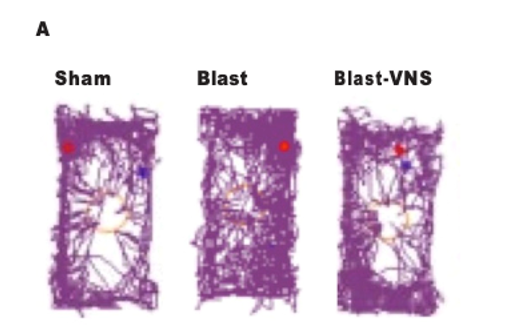
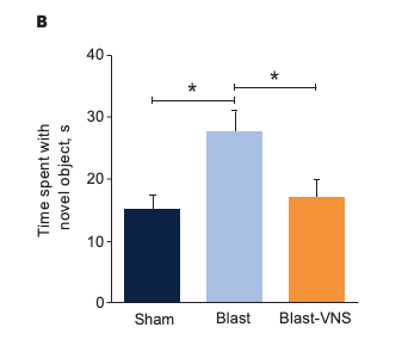
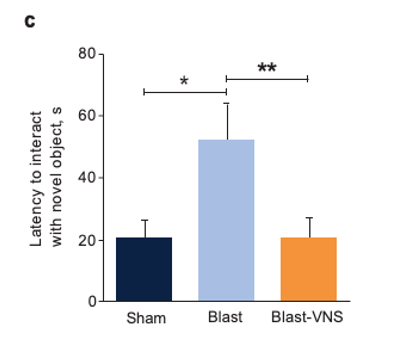
Schindler, et al. Brain Stimuli.28
Abbreviation: VNS, vagus nerve stimulation.
*p<0.05; **p<0.01. Error bars denote standard errors of the mean.
Clinical Evidence
Clinical Evidence Supporting the Utility of nVNS in the Treatment of PTSD
| Reference | Study Population | Main Findings |
|---|---|---|
| Gurel et al. Brain Stimulation. 202029 | Healthy individuals with a history of exposure to trauma |
|
| Gurel et al. Neurobiology of Stress. 202029 | Patients with PTSD |
|
| Bremner et al. Journal of Affective Disorders. 202131 | Patients with PTSD |
|
Abbreviations: CGI-I, Clinical Global Impressions-Improvement; HAM-A, Hamilton Anxiety Autonomic scale; HR, heart rate; IL, interleukin; nVNS, non-invasive vagus nerve stimulation; PAT, pulse arrival time; PEP, post-ejection period; PPG, photoplethysmography; PTSD, posttraumatic stress disorder; RR, respiratory rate.
Effects of nVNS on Physiological Biomarkers of Psychological Stress29
- In healthy participants with a history of psychological trauma, gammaCore™ (nVNS) applied immediately after stress led to significant decreases in peripheral and cardiac sympathetic activity and increases in parasympathetic activity
- The reduction in sympathetic activity and enhancement of parasympathetic activity by nVNS further support its potential clinical utility in patients with PTSD
Primary Outcomes From Physiological Signal Analyses for Stimulation Following Traumatic Stress
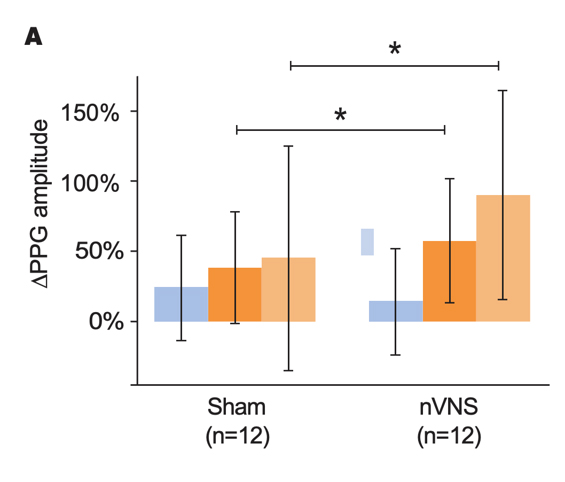
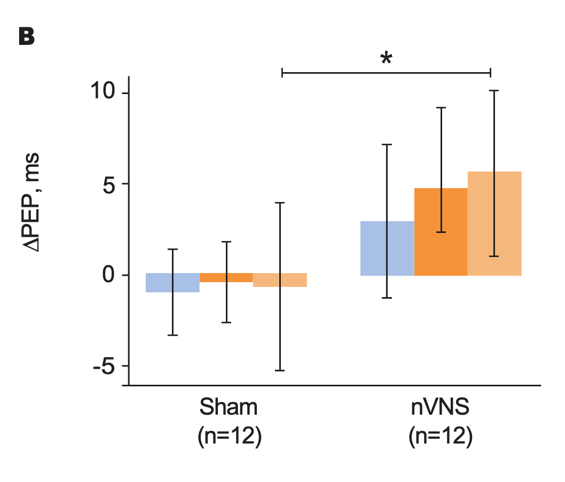
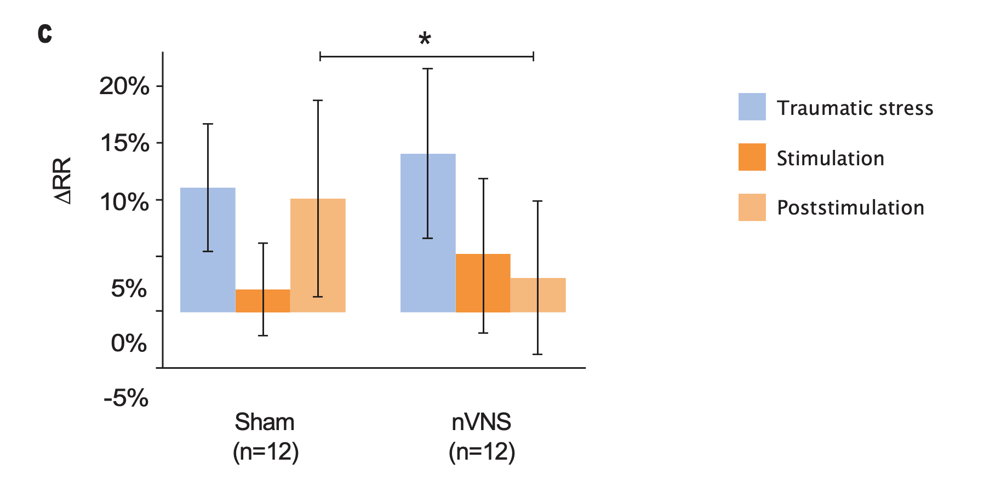
Gurel et al. Brain Stimul. 2020.29
Bars represent the unadjusted mean changes from baseline, error bars: 95% CI, values calculated from raw data, *p<0.05. Abbreviations: PEP, post-ejection period; PPG, photoplethysmography; RR, respiratory rate.
Effects of nVNS on Sympathetic Response to Stress in PTSD30
- Patients with PTSD who were treated with gammaCore™ (nVNS) had decreased sympathetic function during traumatic stress
- nVNS led to significantly greater decreases in long-term heart rate variability over the course of multiple traumatic stress and stimulation protocols
- Attenuation of sympathetic arousal by nVNS in patients with PTSD suggests that nVNS improves the underlying neurobiology in such patients
Outcomes for Active nVNS and Sham Stimulation Following Traumatic Stress in Patients With PTSD
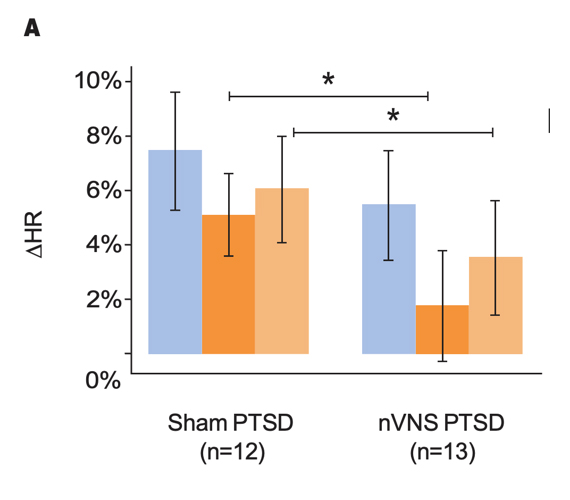
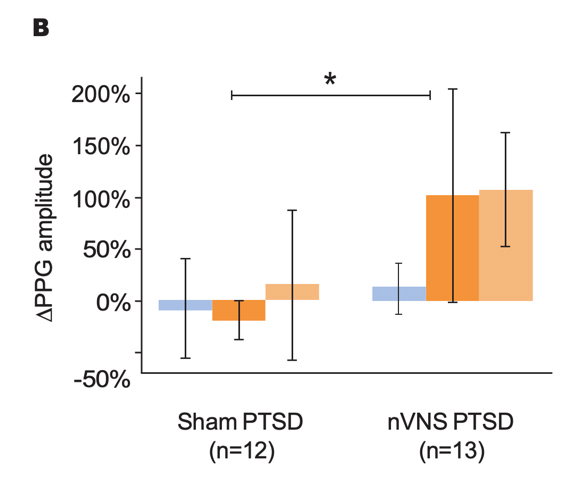
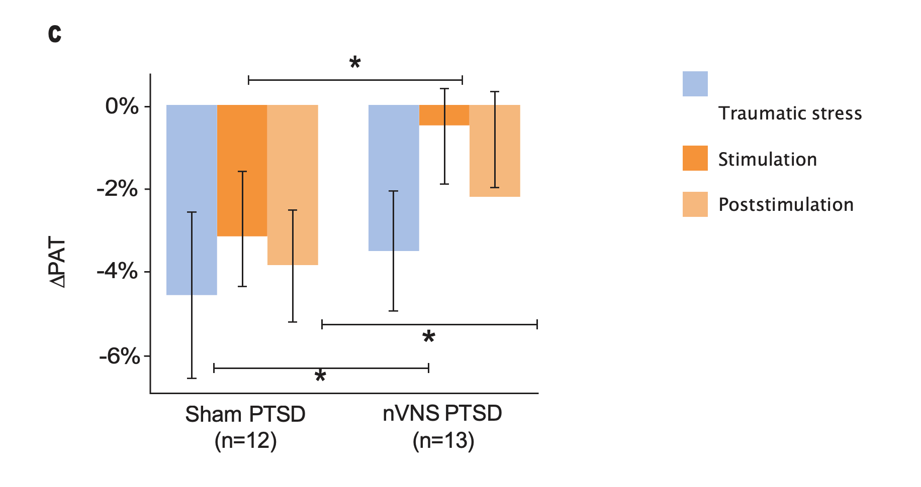
Gurel et al. Neurobiol Stress. 2020.30
Bars represent the unadjusted mean changes from baseline, error bars; 95% CI, values calculated from raw data, *p<0.05. Abbreviations: HR, heart rate; nVNS, noninvasive vagus nerve stimulation; PAT, pulse arrival time; PPG, photoplethysmography; PTSD, posttraumatic stress disorder.
Effects of nVNS on Clinical Symptoms and Inflammatory Biomarkers in PTSD31
- At the end of the 3-month double-blind treatment period, gammaCore™ (nVNS) supported a significant improvement from baseline in PTSD symptoms
- nVNS limited the increases in interleukin-6 levels reported by the sham group at both the beginning and end of the double-blind treatment period
- After approximately 1 month of open-label nVNS therapy, patients who had received sham stimulation during the double-blind period had significant improvements in PTSD symptoms
- nVNS decreased the symptoms of PTSD and the inflammatory response to stress, which could be a component of the mechanisms underlying its beneficial effects on PTSD symptoms
Effects of up to 3 Months of Twice-Daily nVNS or Sham Stimulation on Symptoms of PTSD as Measured With the PCL (A) and the HAM-A Autonomic Scale (B)
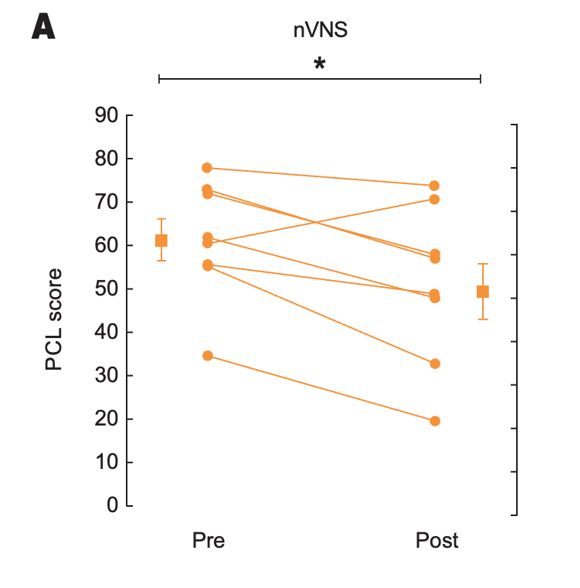
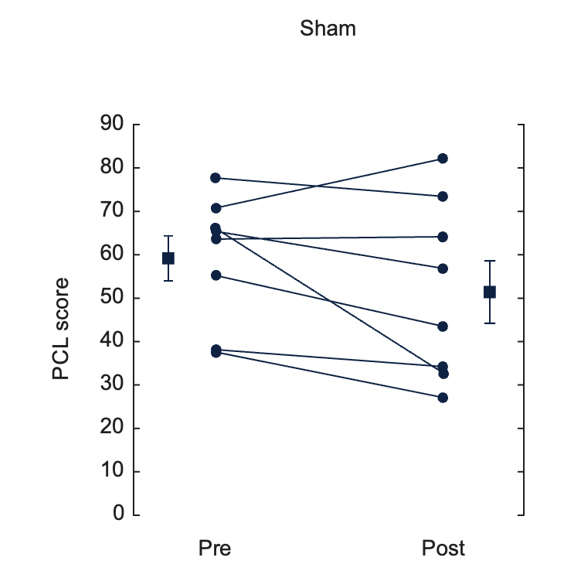
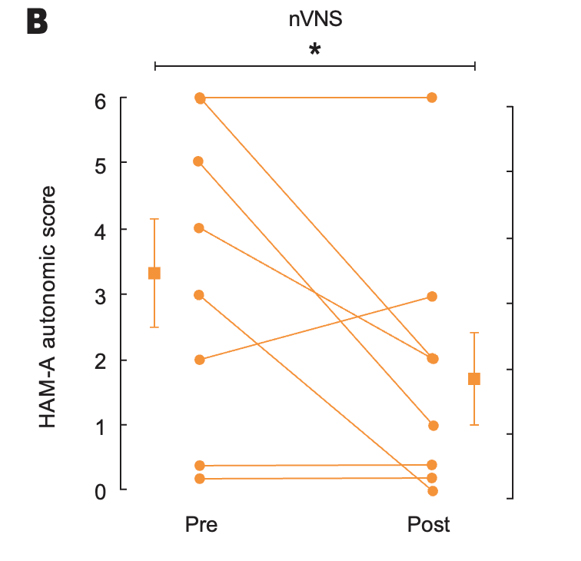
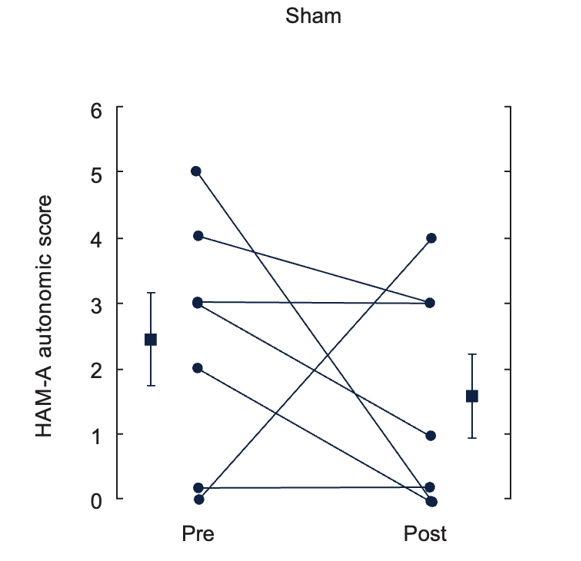
Bremner et al. J Affective Disord. 2021.31
Individual participants are shown with lines separating pre- and posttreatment; lines with bars represent means and SD before and after treatment for both groups. *p<0.05 from pretreatment.
Abbreviations: HAM-A, Hamilton Anxiety Scale; PCL, PTSD checklist; PTSD, posttraumatic stress disorder; nVNS, non-invasive vagus nerve stimulation.
Effects of nVNS and Sham on Clinical Improvement as Measured With the CGI-I in Active and Sham Stimulation Groups at Baseline and 30 and 90 Days After Start of Double-Blind Active (n=9) or Sham (n=11) Treatment in Patients With PTSD
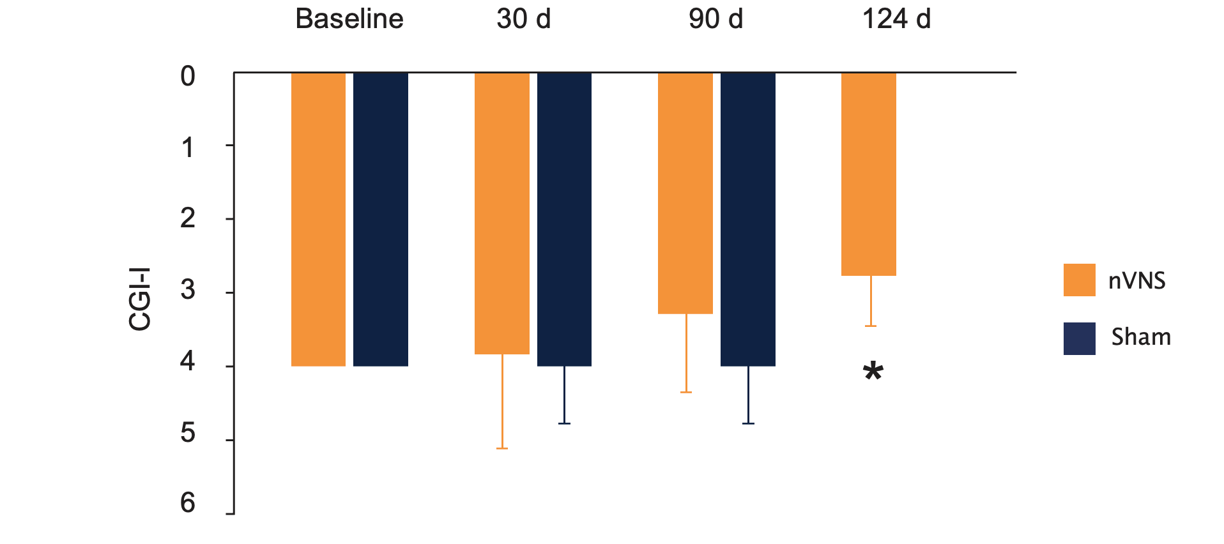
Bremner et al. J Affective Disord. 2021.31
The final measurement at 124 days (34 days after start of open-label treatment) showed a significant improvement compared to after 3 months of sham stimulation. *p=0.003.
Abbreviations: CGI-I, Clinical Global Impressions–Improvement; nVNS, non-invasive vagus nerve stimulation; PTSD, posttraumatic stress disorder.

“Current treatments for PTSD involving medication and psychotherapy have limitations due to limited efficacy, possible side effects, and the unwillingness of many PTSD patients to engage in therapies that involve reliving traumatic memories. gammaCore represents a new class of treatment separate from medication or psychotherapy that is safe, relatively free of side effects, and does not involve costly and invasive procedures for implantation, like previous VNS devices approved by the FDA for treatment of refractory depression.”
J. Douglas Bremner, MD
Emory University School of Medicine
Ongoing and Future Studies
Several studies are underway to further define the efficacy and safety of nVNS for the treatment of PTSD and conditions that are commonly comorbid with PTSD.
Ongoing Studies of nVNS in PTSD and Related Disorders
| Study | Design | Location |
|---|---|---|
| nVNS in posttraumatic stress32 | Randomized, double-blind, sham-controlled | VA San Diego Healthcare System San Diego, CA |
| nVNS in opioid use disorders33 | Randomized, double-blind, sham-controlled | Emory University Atlanta, GA |
| Sympathetic overactivity in PTSD34 | Randomized, double-blind, sham-controlled | Atlanta VA Medical Center Decatur, GA |
Abbreviations: nVNS, non-invasive vagus nerve stimulation; PTSD, posttraumatic stress disorder; VA, Veterans Affairs.
References:
1. Rapaport MH, Clary C, Fayyad R, Endicott J. Quality-of-life impairment in depressive and anxiety disorders. Am J Psychiatry. 2005;162(6):1171-1178. doi:10.1176/appi.ajp.162.6.1171
2. Mayo Clinic. Post-traumatic stress disorder (PTSD). Accessed November 15, 2021. https://www.mayoclinic.org/diseases-conditions/post-traumatic-stressdisorder/symptoms-causes/syc-20355967
3. Sareen J, Stein MB, Friedman M. Posttraumatic stress disorder in adults: epidemiology, pathophysiology, clinical manifestations, course assessment, and diagnosis. Accessed November 15, 2021. https://www.uptodate.com/contents/posttraumatic-stress-disorder-in-adults-epidemiology-pathophysiology-clinical-features-assessment-and-diagnosis
4. Song H, Fang F, Tomasson G, et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA. 2018;319(23):2388-2400. doi:10.1001/jama.2018.7028
5. Peterlin BL, Nijjar SS, Tietjen GE. Post-traumatic stress disorder and migraine: epidemiology, sex differences, and potential mechanisms. Headache. 2011;51(6):860-868. doi:10.1111/j.1526-4610.2011.01907.x
6. Hoppen TH, Priebe S, Vetter I, Morina N. Global burden of post-traumatic stress disorder and major depression in countries affected by war between 1989 and 2019: a systematic review and meta-analysis. BMJ Glob Health. 2021;6(7). doi:10.1136/bmjgh-2021-006303
7. U.S. Department of Veterans Affairs. How common is PTSD in adults? Accessed October 19, 2021.
https://www.ptsd.va.gov/understand/common/common_adults.asp
8. U.S. Department of Veterans Affairs. How common is PTSD in veterans? Accessed November 15, 2021.
https://www.ptsd.va.gov/understand/common/common_veterans.asp
9. Lippa SM, Fonda JR, Fortier CB, et al. Deployment-related psychiatric and behavioral conditions and their association with functional disability in OEF/OIF/OND veterans. J Trauma Stress. 2015;28(1):25-33. doi:10.1002/ jts.21979
10. Bremner JD, Gurel NZ, Wittbrodt MT, et al. Application of noninvasive vagal nerve stimulation to stress-related psychiatric disorders. J Pers Med. 2020;10(3). doi:10.3390/jpm10030119
11. Annoni EM, Van Helden D, Guo Y, et al. Chronic low-level vagus nerve stimulation improves long-term survival in salt-sensitive hypertensive rats. Front Physiol. 2019;10:25. doi:10.3389/fphys.2019.00025
12. Cao J, Lu KH, Powley TL, Liu Z. Vagal nerve stimulation triggers widespread responses and alters large-scale functional connectivity in the rat brain. PLoS One. 2017;12(12):e0189518. doi:10.1371/journal.pone.0189518
13. Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 2014;7(6):871-877. doi:10.1016/j.brs.2014.07.031
14. Farrand AQ, Verner RS, McGuire RM, Helke KL, Hinson VK, Boger HA. Differential effects of vagus nerve stimulation paradigms guide clinical development for Parkinson’s disease. Brain Stimul. 2020;13(5):1323-1332. doi:10.1016/j. brs.2020.06.078
15. Follesa P, Biggio F, Gorini G, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28-34. doi:10.1016/j.brainres.2007.08.045
16. Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res. 2013;207:275-299. doi:10.1016/B978-0-444-63327-9.00010-2
17. Hulsey DR, Hays SA, Khodaparast N, et al. Reorganization of motor cortex by vagus nerve stimulation requires cholinergic innervation. Brain Stimul. 2016;9(2):174-181. doi:10.1016/j.brs.2015.12.007
18. Liu Y, Tao T, Li W, Bo Y. Regulating autonomic nervous system homeostasis improves pulmonary function in rabbits with acute lung injury. BMC Pulm Med. 2017;17(1):98. doi:10.1186/s12890-017-0436-0
19. Mondal B, Choudhury S, Banerjee R, et al. Non-invasive vagus nerve stimulation improves clinical and molecular biomarkers of Parkinson’s disease in patients with freezing of gait. NPJ Parkinsons Dis. 2021;7(1):46. doi:10.1038/ s41531-021-00190-x
20. Silberstein SD, Yuan H, Najib U, et al. Non-invasive vagus nerve stimulation for primary headache: a clinical update. Cephalalgia. 2020;40(12):1370-1384. doi:10.1177/0333102420941864
21. Tarn J, Legg S, Mitchell S, Simon B, Ng WF. The effects of noninvasive vagus nerve stimulation on fatigue and immune responses in patients with primary Sjogren’s syndrome. Neuromodulation. 2019;22(5):580-585. doi:10.1111/ner.12879
22. Vida G, Pena G, Deitch EA, Ulloa L. alpha7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J Immunol. 2011;186(7):4340-4346. doi:10.4049/jimmunol.1003722
23. Hersman S, Hoffman AN, Hodgins L, et al. Cholinergic signaling alters stress-induced sensitization of hippocampal contextual learning. Front Neurosci. 2019;13:251. doi:10.3389/fnins.2019.00251
24. Noble LJ, Meruva VB, Hays SA, Rennaker RL, Kilgard MP, McIntyre CK. Vagus nerve stimulation promotes generalization of conditioned fear extinction and reduces anxiety in rats. Brain Stimul. 2019;12(1):9-18. doi:10.1016/j.brs.2018.09.013
25. Pena DF, Childs JE, Willett S, Vital A, McIntyre CK, Kroener S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front Behav Neurosci. 2014;8:327. doi:10.3389/fnbeh.2014.00327
26. Souza RR, Robertson NM, Pruitt DT, et al. Vagus nerve stimulation reverses the extinction impairments in a model of PTSD with prolonged and repeated trauma.Stress. 2019;22(4):509-520. doi:10.1080/10253890.2019.1602604
27. Schindler AG, Meabon JS, Baskin B, et al. Non-invasive vagus nerve stimulation for the prevention/treatment of comorbid mild traumatic brain injury and PTSD. Poster presented at: 3rd International Brain Stimulation Conference; February 24-27, 2019; Vancouver, BC, Canada.
28. Schindler AG, Meabon JS, Baskin B, et al. Non-invasive vagus nerve stimulation for the prevention/treatment of comorbid mild traumatic brain injury and PTSD. Brain Stimul. 2019;12(2):P418-P419. doi:10.1016/j.brs.2018.12.356
29. Gurel NZ, Huang M, Wittbrodt MT, et al. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul. 2020;13(1):47-59. doi:10.1016/j. brs.2019.08.002
30. Gurel NZ, Wittbrodt MT, Jung H, et al. Transcutaneous cervical vagal nerve stimulation reduces sympathetic responses to stress in posttraumatic stress disorder: A double-blind, randomized, sham controlled trial. Neurobiol Stress. 2020;13:100264. doi:10.1016/j.ynstr.2020.100264
31. Bremner JD, Wittbrodt MT, Gurel NZ, et al. Transcutaneous cervical vagal nerve stimulation in patients with posttraumatic stress disorder (PTSD): a pilot study of effects on PTSD symptoms and interleukin-6 response to stress. J Affective Disord. 2021;6:100190. doi:10.1016/j.jadr.2021.100190
32. Vagal nerve stimulation to probe inflammation and brain in post-traumatic stress. Updated March 1, 2021. Accessed November 30, 2021.
https://clinicaltrials.gov/ct2/show/ NCT03858985?term=lerman%2C+san+diego&draw=2&rank=2
33. Non-invasive vagal nerve stimulation in opioid use disorders (nVNS in OUDs). Updated September 17, 2021. Accessed November 18, 2021.
https://clinicaltrials.gov/ct2/show NCT04556552?term=gammacore&draw=4&rank=29
34. Sympathetic overactivity in post-traumatic stress disorder (SO-PTSD). Updated July 15, 2021. Accessed November 18, 2021.
https://clinicaltrials.gov/ct2/show/NCT01627301?term=gammacore&draw=4&rank=24

Our history
Founded on the interest in the pioneering concept of using vagus nerve stimulation to improve patient outcomes in a variety of conditions.