
Our pipeline
We are advancing a pipeline that builds on our expertise in nVNS therapy. Take a look at what we’re working on and where it is in development.
US pipeline clinical development
The development plan draws on a significant amount of pre-clinical and clinical work that has been generated over the past several decades by researchers using VNS technologies.
Preclinical/Pilot Trials
We work with various institutes and organizations to investigate what disorders nVNS could impact.
Pivotal Trials
Studies intended to provide scientific evidence of the safety and efficacy of gammaCore in a particular disease and population.
FDA Clearance
FDA reviews clinical data and determines whether to clear gammaCore for commercialization.
Commercial Launch
Marketing of gammaCore to the intended audience.
Commercialized indications for Europe
gammaCore is indicated for the following conditions:


CE marks for the EU/EFTA/EEA and UK
-
Treatment or prevention of symptoms of reactive airway disease, including asthma, bronchoconstriction, exercise-induced bronchospasm, and COPD.
-
Acute and/or prophylactic treatment of primary headache (migraine, cluster headache, and hemicrania continua) and medication overuse headache in adults.
-
Adjunctive therapy for adults to reduce the symptoms of certain anxiety and depression conditions (including panic disorder, posttraumatic stress disorder, obsessive-compulsive disorder, and major depressive disorder).
-
Adjunctive therapy in the prevention of partial onset and generalized seizures associated with epilepsy in adults.
-
Adjunctive therapy for adults to reduce the symptoms of gastric motility disorders and irritable bowel syndrome (including nausea, vomiting, bloating/distention, early satiety, and abdominal pain).
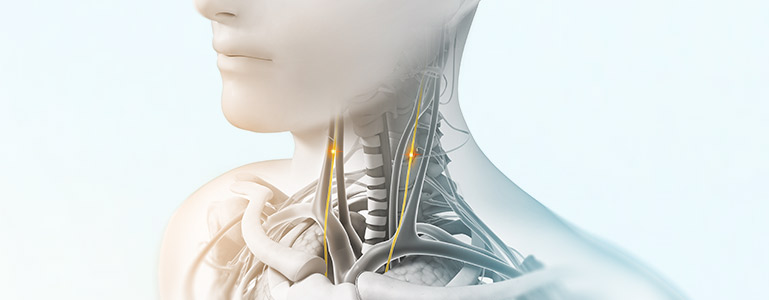
nVNS therapy
Vagus nerve stimulation is a breakthrough technology that helps control multiple disorders.
