In the news

electroCore Completes $70 Million Series B Funding Round
Investment to fund commercialization of gammaCore® and further clinical development of nVNS Basking Ridge, NJ, November 29, 2017 – electroCore, a U.S.-based bioelectronic medicine healthcare company advancing better patient therapies through superior approaches to neuromodulation, announced today it completed its Series B financing that has brought in just over $70 million of capital to the company. The financing was led by Core Ventures II, an investment group that previously participated in the Company’s Series A round. The Series B financing also included significant participation from another Series A investor, Merck’s Global Health Innovation Fund, a venture capital arm of Merck & Co. Inc. Investors participating in recent Series B closings included Gakasa, an affiliate of Knoll Capital Management, as well as American Investment Holdings LLC and the Vinik Family Foundation, both of which are affiliated with Jeffrey Vinik, former manager of the Fidelity Magellan Fund. Paulson Investment Company assisted electroCore with this financing. The proceeds of this Series B financing will be used for the commercial launch and expansion via scientific exploration of the company’s gammaCore therapy into the primary headache market, as well as the continued clinical and scientific development of the company’s non-invasive, vagus nerve stimulation (nVNS) therapy....
electroCore Announces Results from gammaCore® (non-invasive vagus nerve stimulator) Study in Migraine at the 18th Congress of the International Headache Society
PRESTO demonstrates clinical benefit of gammaCore in the acute treatment of migraine compared to sham Basking Ridge, NJ, September 11, 2017 – electroCore, a neuroscience and technology company dedicated to improving patient outcomes through technological advancement, announced the first results from the PRESTO (The PRospectivE Study of nVNS for the Acute Treatment Of Migraine) clinical trial evaluating the use of gammaCore® (non-invasive vagus nerve stimulator) in migraine at the 18th Congress of the International Headache Society (IHC). Findings from the study “Non-invasive Vagus Nerve Stimulation (nVNS) for the Acute Treatment of Migraine: A Randomized Controlled Trial” (IHC Abstract OC-LB-002) were presented on Saturday, September 9 as a late-breaking oral presentation. “Migraine is the third most common disease in the world,[i] and one of the 10 most disabling diseases, which highlights a need for novel treatment options,” said Dr. Cristina Tassorelli, Director of the Headache Science Centre, National Neurological Institute C. Mondino Foundation; Professor at the University of Pavia, Pavia, Italy; and principal trial investigator. “The PRESTO data suggests that gammaCore was rapidly effective, well tolerated and practical for the acute treatment of episodic migraine. The data supports the use of gammaCore to successfully treat a migraine, making the device a...

Publicis Health CEO Joins electroCore’s Board of Directors
Nick Colucci Brings Invaluable Healthcare Communications Expertise to Enhance electroCore’s Continued Growth Basking Ridge, NJ, August 29, 2017 – electroCore, a neuroscience and technology company dedicated to improving patient outcomes through technological advancement, today announced that Nick Colucci, Chief Executive Officer (CEO) of Publicis Health, has been elected to join electroCore’s Board of Directors, effective immediately. “Nick brings more than 30 years of experience across important life sciences and communications industries; he is a true leader and visionary in the field,” said Francis R. Amato, Chief Executive Officer of electroCore. “We are thrilled to welcome him, appreciate his willingness to serve as a director and look forward to benefitting from his expert insight and counsel.” Mr. Colucci, who has served as Publicis Health CEO since May 2007, previously worked as the company’s Chief Operating Officer and joined in 2000 as President. His experience spans both the client and agency sides of the communications business. Prior to joining Publicis, Mr. Colucci served as the Vice President of Marketing & Sales at EyeSys Technologies and Marketing Director at Roche. In addition to his current role, Mr. Colucci leads key initiatives for the Coalition for Healthcare Communication, an industry trade association, and serves...
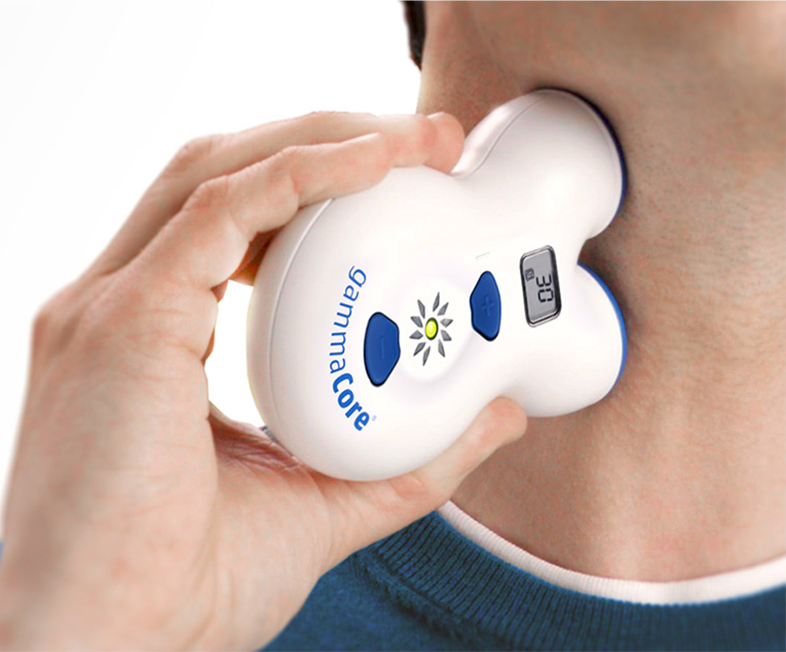
gammaCore®, the First Non-Invasive Vagus Nerve Stimulator Applied at the Neck, Now Available for Adult Patients in the U.S.
electroCore launches gammaCore Patient Registry (GPR) to provide access to hand-held, easy-to-use device for the acute treatment of pain associated with episodic cluster headache in adults Basking Ridge, NJ, July 18, 2017 – electroCore, a neuroscience and technology company dedicated to improving patient outcomes through technological advancement, announced today the commercial launch of gammaCore® (non-invasive vagus nerve stimulator) for the acute treatment of pain associated with episodic cluster headache in adult patients in the United States (U.S.). gammaCore is the first non-invasive, hand-held medical device applied at the neck and sends gentle, patented electrical stimulation through the skin to activate the vagus nerve, resulting in the reduction of pain. “We are proud to bring gammaCore to the U.S. market, as it provides episodic cluster headache sufferers with a much-needed treatment option for this rare and often debilitating condition,” said Francis R. Amato, Chief Executive Officer of electroCore. “The commercial launch also signifies an important milestone for electroCore, marking the first marketed product for the company in the U.S.” Patients can receive the device by enrolling in gammaCore Patient Registry (GPR), a non-research registry intended to examine the patient experience with gammaCore. As part of the registry, those...

electroCore Announces Appointment of New Chief Medical Officer
Peter Staats MD, MBA, ABIPP, FIPP Joins electroCore to Lead Global Development of Company’s Non-invasive Vagus Nerve Stimulation (nVNS) Therapy Basking Ridge, NJ, June 21, 2017 – electroCore, a neuroscience and technology company dedicated to improving patient outcomes through technological advancement, today announced the appointment of Peter Staats MD, MBA, ABIPP, FIPP as Chief Medical Officer (CMO) of electroCore. Dr. Staats brings with him more than 25 years’ experience in the medical field focused in the area of pain management and has an international reputation for successfully developing and implementing minimally invasive procedures for chronic pain and neuromodulation. The appointment of Dr. Staats to CMO, which was unanimously approved by the company’s Board, is part of electroCore’s evolution and furthers the momentum toward future growth. Dr. Staats will be responsible for leading electroCore’s global medical and scientific efforts, focused on the development of non-invasive vagus nerve stimulation (nVNS) for the treatment of primary headaches (cluster headache and migraine) and other conditions in neurology, immunology and endocrinology. He will join electroCore’s management team, reporting to Francis R. Amato, the company’s Chief Executive Officer. “We are thrilled with the appointment of Dr. Staats as CMO at electroCore and know that...

electroCore CEO to Join Company’s Board of Directors
Francis R. Amato to Take on Additional Role to Help Further Position the Company for Growth Basking Ridge, NJ, June 21, 2017 – electroCore, a neuroscience and technology company dedicated to improving patient outcomes through technological advancement, today announced that Chief Executive Officer (CEO), Francis R. Amato, has been elected to join the company’s Board of Directors effective immediately. Mr. Amato will also remain in his role as CEO of electroCore. “Frank is a talented executive who has demonstrated strong business acumen and stewardship of growth as electroCore advances the company’s proprietary non-invasive vagus nerve stimulation (nVNS) therapy,” said J.P. Errico, Founder, Principal Investor and Chief Science & Strategy Officer of electroCore. “With this appointment, we are excited to expand his role and further benefit from his leadership.” Mr. Amato, who has served as CEO since July 2016 and joined electroCore in 2012 as Chief Operating Officer (COO), has extensive commercial experience in the pharmaceutical industry. Prior to joining electroCore, Mr. Amato held the position of US Vice President, Specialty Commercial Operations Group, at Merck. Other senior positions held include Executive Director, Global Business Operations, at Schering-Plough; Business Unit Lead, Oncology for Ligand Pharmaceutical; and National Accounts Director, Specialty...

electroCore Receives 510(k) Clearance for gammaCore-S® (non-invasive vagus nerve stimulator) for the Acute Treatment of Pain Associated with Episodic Cluster Headache in Adult Patients
Regulatory clearance marks most recent achievement in new device being available to the approximately 400,000 Americans living with episodic cluster headaches Basking Ridge, NJ, June 15, 2017 – electroCore, a neuroscience and technology company dedicated to improving patient outcomes through technological advancement, announced today that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to market a newer version of gammaCore® (non-invasive vagus nerve stimulator) – the gammaCore-S® – for the acute treatment of pain associated with episodic cluster headache (eCH) in adult patients. gammaCore transmits a mild electrical stimulation to the vagus nerve through the skin to block the pain signals that cause eCH. “This clearance represents a significant milestone for electroCore as we are now one step closer to bringing gammaCore to patients in the U.S.,” said Francis R. Amato, Chief Executive Officer of electroCore. “By being able to market this version of gammaCore in the U.S., adult patients will now have access to a more user-friendly device, with the latest technology to treat pain associated with episodic cluster headache.” gammaCore was released by the FDA for the acute treatment of pain associated with eCH in adult patients on April 14, 2017. gammaCore-S has...
New Analysis of ACT Clinical Trial Program Highlights Efficacy and Safety of electroCore’s gammaCore® (non-invasive vagus nerve stimulator) in the Acute Treatment of Episodic Cluster Headache
Data from Pooled Analysis of ACT1 and ACT2 To Be Presented in Late-Breaking Oral Session at Annual Scientific Meeting of the American Headache Society Basking Ridge, NJ, June 8, 2017 – electroCore, a neuroscience and technology company dedicated to improving patient outcomes through technological advancement, today announced new data from the ACT (Non–Invasive Vagus Nerve Stimulation for the ACute Treatment of Cluster Headache) clinical trial program demonstrating the safety and efficacy of gammaCore® (non-invasive vagus nerve stimulator) as an acute treatment for patients with episodic cluster headache (eCH) at the 59th Annual Scientific Meeting of the American Headache Society (AHS). Results of the pooled analysis, “Non-invasive Vagus Nerve Stimulation for Acute Treatment of Episodic and Chronic Cluster Headache: Pooled Analysis of Data From Two Randomized, Double-blind, Sham-Controlled Clinical Trials” (AHS Abstract #IOR3), will be presented on Saturday, June 10th as a late-breaking oral abstract. “The findings from this pooled analysis of ACT1 and ACT2 are exciting,” said Professor Peter Goadsby, MD, PhD, Professor of Neurology, King’s College London and Director, NIHR-Wellcome Trust Clinical Research Facility, King’s College Hospital, London. “The data not only reinforces the safety and efficacy of gammaCore but also highlights some of the important advantages of non-invasive...

electroCore Announces Data Presentation at the 69th American Academy of Neurology Annual Meeting
Data from ACT2 Clinical Trial of gammaCore® in Cluster Headache Presented for First Time Yesterday During Emerging Science Session Basking Ridge, NJ, April 26, 2017 – electroCore, a neuroscience and technology company dedicated to improving patient outcomes through technological advancement, announced today that “Non-invasive Vagus Nerve Stimulation for the Acute Treatment of Episodic and Chronic Cluster Headache: Findings from the Randomized, Double-blind, Sham-controlled ACT2 Study,” was presented yesterday at the 69th American Academy of Neurology (AAN) Annual Meeting, being held April 22-28, 2017, in Boston, MA. On behalf of the authors, including primary investigator, Peter Goadsby, MD, PhD, Professor of Neurology, King’s College London and Director, NIHR-Wellcome Trust Clinical Research Facility, King’s College Hospital, London, Dr. Ilse de Coo, MD, Leiden University Medical Centre presented the data from the ACT2 clinical trial (Non-Invasive Vagus Nerve Stimulation for the ACute Treatment of Cluster Headache), evaluating the use of gammaCore® (non-invasive vagus nerve stimulator) for the acute treatment of pain associated with episodic or chronic cluster headache (eCH or cCH, respectively). Results presented yesterday demonstrated that the proportion of all attacks that achieved pain-free status at 15 minutes (primary endpoint) was superior for those using gammaCore (47.5%) versus sham (6.2%; p < 0.01) in...

FDA Releases gammaCore®, the First Non-Invasive Vagus Nerve Stimulation Therapy Applied at the Neck for Acute Treatment of Pain Associated with Episodic Cluster Headache in Adult Patients
First U.S. Food and Drug Administration (FDA) release for electroCore, a neuroscience and technology company – Hand-held, non-invasive, easy-to-use device provides new option for the approximately 400,000 Americans living with this rare but extremely debilitating headache disorder Basking Ridge, NJ, April 18, 2017 – electroCore, a neuroscience and technology company dedicated to improving patient outcomes through technological advancement, announced today that the U.S. Food and Drug Administration (FDA) released the use of gammaCore® (non-invasive vagus nerve stimulator) for the acute treatment of pain associated with episodic cluster headache in adult patients. gammaCore transmits a mild electrical stimulation to the vagus nerve through the skin, resulting in a reduction of pain. This is the first FDA product release for electroCore in the U.S. “Cluster headache is a rare, debilitating and difficult to treat disorder with few effective acute therapies,” said Stephen Silberstein, MD, Director, Headache Center, Jefferson University, Philadelphia, PA. “The FDA release of gammaCore is an important advance in the treatment of the pain associated with cluster headache. It is a way for patients to treat their symptoms as often as they need to use the device. It does not have the side effects or dose limitations of commonly prescribed...

National Institute of Health Research (NIHR) publication reinforces potential role of gammaCore in helping UK patients manage medically unexplained symptoms (MUS)
electroCore welcomes the recent NIHR technology alert on gammaCore, which profiles the CE-marked device’s role in helping patients in the UK manage long-term neurological conditions such as migraine and medically unexplained symptoms. The alert, which is designed to notify clinicians and those involved in the commissioning of healthcare products or services of the availability of new technologies, highlights the potential for gammaCore to enhance the quality of life for people with long-term conditions, thereby aligning with this domain within the NHS Outcomes Framework. Based upon independent research funded by the NIHR, and published within the NIHR’s Horizon Scanning Research and Intelligence Centre (HSRIC), this alert bolsters the Company’s belief in the potential of gammaCore to help alleviate some of the unique pressures that exist within the UK’s healthcare system. By empowering patients to safely and effectively self-manage their medically unexplained symptoms at home, it is anticipated that usage of the device will help to reduce the workload demand currently placed on GPs and the wider health and social care system. gammaCore received its first CE mark in 2011, and a positive review from the Interventional Procedures Advisory Committee of the National Institute for Health and Care Excellence (NICE), on its...

Mount Sinai Beth Israel Receive Award from The United States Department of Defense to Study Gulf War Illness with electroCore’s non-invasive vagus nerve stimulation therapy
The Pain & Fatigue Study Center at Mount Sinai Beth Israel in New York has been awarded a United States Army Medical Research grant to conduct a study into the treatment of veterans of the 1990-1991 Gulf War who have Gulf War Illness using electroCore’s non-invasive vagus nerve stimulation (nVNS) therapy gammaCore. The $703,272 grant will be used to fund a randomized clinical trial involving more than 40 veterans with widespread pain and migraines, a common complaint of gulf war veterans. This study will be led by Benjamin Natelson MD, an internationally recognized leader in clinical care and research into medically unexplained pain and fatigue. The trial will be conducted at the Pain & Fatigue Study Center at Mount Sinai Beth Israel in Manhattan. “While pharmaceutical treatments are available for the treatment of Gulf War Illness, they have drawbacks,” says Dr. Natelson. “These drugs don’t work for all patients, the effect frequently only lasts a couple of months and the side effects are often so severe as to preclude their use so there is a real need for further research into treatment of this debilitating condition.” Having been previously involved with surgically implanted vagus nerve stimulation (VNS), in a fibromyalgia...